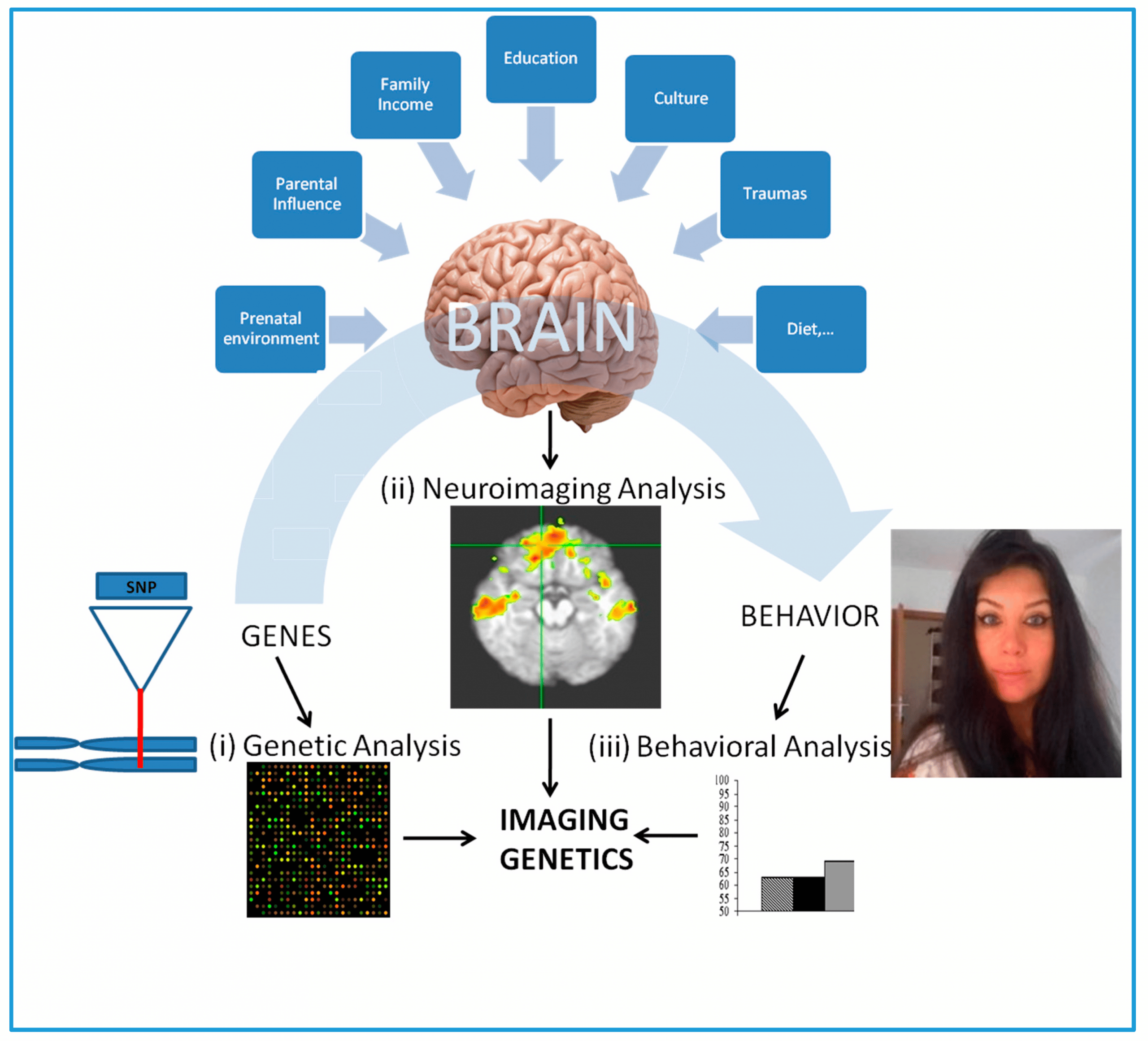
The Thickness And Size Of The Surface Of The Brain May Be Promising Biomarkers
For a long time, researchers have tried to find out if there are so-called biomarkers for mental disorders such as schizophrenia. A biomarker for schizophrenia would mean that we could determine someones risk of schizophrenia based on one or more measurable biological characteristics.
Today, it is not possible to determine conclusively whether someone has schizophrenia based on medical examinations. We want to find out if there is a reliable and objective biomarker, i.e., one or more biological characteristics, that are associated with the risk of schizophrenia, Weiqiu Cheng states.
She adds:
The thickness and size of the surface of the brain can potentially be such biomarkers.
The findings from the present study may be a small step towards this endeavour.
The genetic overlap that we found help us understand the genetic relationship between schizophrenia and the thickness and size of the surface of the brain. This may pave the way for future biomarker studies of schizophrenia, Cheng points out.
Premorbid Cognitive And Scholastic Performance
Schizophrenia patients, when considered as a group, have intellectual impairments, some of which predate the onset of psychotic symptoms. Individuals who later develop schizophrenia have been found to perform below average on standardized measures of intelligence in childhood, adolescence and young adulthood, and to show lower premorbid IQ than the general population The lower the IQ, the higher is the risk for later development of schizophrenia.
Poor school performance can be seen as a premorbid sign. Repeating a grade, difficulties in completing the final level of schooling, and social and behavioural difficulties have also been found to be risk factors for developing schizophrenia. In the Northern Finland 1966 Birth Cohort, 14-year-olds who were below their expected normal grade were three times more likely to develop schizophrenia than those in their normal grade, but low school marks did not predict schizophrenia. Developmental continuity, indicated by early developmental deviation in the first year of life associated with lower school performance at age 16 years, has been found to be stronger among children who develop psychoses later in life than among normal controls and those admitted to hospital for non-psychotic psychiatric disorder.
Chromosome 1q42 Candidate Genes
DISC1
Ongoing work in the Scottish pedigree has now identified three genes disrupted by the breakpoint, one of which, disrupted in schizophrenia 1 , has been intensively studied. Studies of this locus in samples with no translocation have been mixed, but positive results, , , outnumber negative ones., , A frameshift mutation has also been identified in a single multiply affected family.DISC1 appears to have a role in cytoskeletal regulation, and thus may affect neuronal migration, neurite outgrowth and intracellular transport.,
You May Like: Can Depression Cause Back Pain
Traumatic Experiences Early In Life
A have suggested a correlation between traumatic experiences in childhood and adolescence and the development of schizophrenia in people with a genetic risk.
For people with a genetic predisposition to schizophrenia, the risk of onset for this condition is
One thing to keep in mind is that insufficient vitamin D during pregnancy isnt always associated with access to nutritious foods. Instead, it can be related to less sunlight and time outdoors.
Because of this, the review noted that children born in the winter and spring carry a slightly higher genetic risk of schizophrenia.
Early Days: Linkage Candidate Genes And Lack Of Replication
The polygenic nature of schizophrenia has been suspected and debated for a long time . Hoping that at least some families might segregate a single disease-causing variant, or that the overall number of such variants is limited, numerous linkage studies have tested both parametric and non-parametric approaches. Starting as early as 1972, Elston et al. reported possible linkage of schizophrenia with specific blood groups, and many other linkage studies followed. Unfortunately, most were met with disappointment, almost always showing weak results and often failing to replicate one another. The same was true for the first association studies that focused on candidate genes or followed up previous linkage results. At the time, we did not appreciate the large number of risk variants underlying schizophrenia and the small contribution these variants have on the risk. The studies of the era were vastly underpowered and often produced no or false positive results. Only now that we have succeeded in identifying true schizophrenia risk variants have we come to appreciate the serious limitations of earlier work. Very few of the early gene findings remain under investigation today, and those that do are not because of robust evidence for a role in the disease, but rather because of continuing interest in their function revealed by the work initially triggered by the associations.
Read Also: What Are The Top Phobias
Current Issues And Challenge
The history of the search for genes contributing liability to schizophrenia is around a quarter of century old, but it is always dashed with nonreplication of the finding. This has been so despite consistent evidence from family, twin and adoption studies of an important genetic contribution the heritability of schizophrenia is estimated to be approximately 80% . The reasons for the difficulty in finding genes include the complexity of the phenotype, heterogeneity and lack of biological marker. The mode of transmission is multifactorial where non-genetic determinants are also operating. As has been pointed earlier, schizophrenia does not conform to a classical Mendelian pattern of inheritance and it is now clear that most, perhaps all, cases involve the combined effects of many genes, each conferring a small increase in liability to the disorder not due to single gene of major effects. As a consequence, a single gene does not seem to cause the disorder thus no causal disease genes, only susceptibility genes are operating. Otherwise a consistently replicable linkage signal should have been detected. Advancement has also been hampered by the relatively small size of many studies. Not only are large sample needed to detect small effects, but even larger samples are needed to replicate positive findings
Chromosome 13q14q32 Candidate Genes
G72 and DAAO
An elegant recent study examined markers in the distal 5 Mb of this broad linkage region, site of one of the most significant findings on chromosome 13. Nearly 200 SNPs were tested across the region and identified two regions of association. In one of these regions, two genes and G30 were investigated. Of note, the exons of these genes could not be predicted by any computational method tested, suggesting that they are highly novel in their sequence and organization. Both genes show alternative transcripts in the brain and other tissues. Association studies of SNPs within G72 have not yet provided a clear pattern. One of these is nonsynonymous and is significant alone. The nature of the amino-acid change is conservative, but has major functional consequences in some proteins. However, the overall pattern of results is probably most consistent with the existence of further unidentified predisposing variants in this gene.
D-amino acid oxidase is activated by the protein product of G72. Four SNPs in the DAO gene on 12q24 were significantly associated. Results of this kind are rare, so this study had a unique opportunity to test for an epistatic genetic interaction. Evidence for epistasis was observed for one pair of DAAO and G72 genotypes, supporting a potential interaction between them in risk for schizophrenia. Replication studies have generally provided confirmation of a role for G72., , , , , Only one negative report has appeared.
Read Also: How To Help Someone Schizophrenia
Overlap With Other Disorders
Several studies have suggested a genetic overlap and possible genetic correlation between schizophrenia and other psychiatric disorders including autism spectrum disorder, attention deficit hyperactivity disorder, bipolar disorder, and major depressive disorder. One genome-wide association study analyzed single-nucleotide polymorphism data for the five disorders four gene areas overlapped with the five disorders, two of which regulate calcium balance in the brain.
Genetic Factors In Schizophrenia
Contrary to common belief, a split personality is not one of the potential symptoms of schizophrenia. “Persons affected with the illness, however, may exhibit changes in thought and behaviour,” explains Dan Rujescu, head of the Molecular and Clinical Neurobiology Section at the Department of Psychiatry, University of Munich. “Hallucinations or delusions are only a few of the potential symptoms of this severe mental illness.” Symptoms may also include a blunted affect along with episodes of depression. Depending on the manifestation, schizophrenia is classified into different subtypes. Altogether, the illness is among the most severe mental illnesses known. According to estimates, approximately one in every hundred persons experiences a schizophrenic episode at least once in their lives. In Germany alone there are approximately 800,000 persons affected with the illness. It is still only possible to partially treat schizophrenia. In a best case scenario, such treatment can offer a patient a good level of functioning despite the illness.
Don’t Miss: Will Er Prescribe Anxiety Meds
The Default Mode Network
When weâre just hanging out — the dishes are done, weâve finished our homework, or we’ve completed a tough project at work — our thoughts are free to roam. This âdefault modeâ allows us time to daydream, reflect, and plan. It helps us process our thoughts and memories. Scientists call this the default mode network. When weâre not focused on a given task, it âlights up.” If you have schizophrenia, your default mode network seems to be in overdrive. You may not be able to pay attention or remember information in this mode, one study shows.
Pregnancy And Delivery Complications
The examination of developmental abnormalities such as pregnancy and delivery complications, especially in conjunction with genetic risk factors, has provided useful information on precursor states for schizophrenia . For example, patients with schizophrenia have experienced a greater number of labour and delivery complications at birth than have normal controls . Among these complications is pre-eclampsia, which results in foetal hypoxia, and leads to a nine-fold increase in the risk for subsequent schizophrenia . Cannon found a dose-dependent relationship between risk of schizophrenia and severity of perinatal hypoxia. Interestingly, the same researchers found that among the offspring of mothers with schizophrenia, rates of birth complications were higher in the group who eventually developed schizophrenia than in the group who did not, or who developed schizotypal personality disorder. Moreover, birth complications were unrelated to the development of schizophrenia in a control, low-risk group whose parents did not have schizophrenia.
Findings from the Philadelphia cohort of the National Collaborative Perinatal Project indicated that the risk of developing schizophrenia among children of parents with schizophrenia increased as a function of the number of hypoxiarelated birth complications . Hypoxia-related birth complications, but not pregnancy and other delivery complications, predicted subsequent schizophrenia among children of patients with schizophrenia.
You May Like: Can Anxiety Lead To Depression
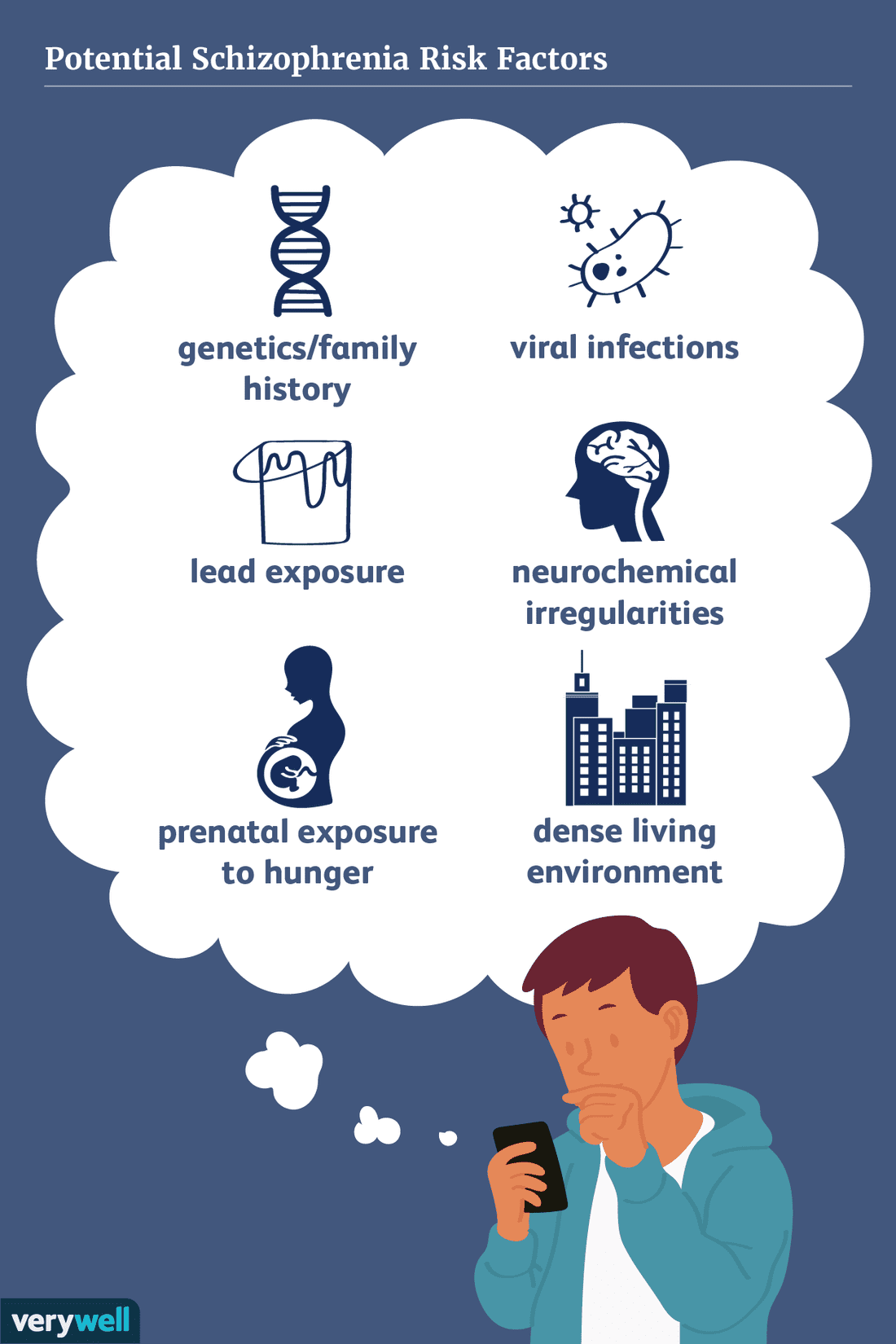
Risk Factors Of Schizophrenia
Risk factors of schizophrenia include many genetic and environmental phenomena. The prevailing model of schizophrenia is that of a special neurodevelopmental disorder with no precise boundary or single cause . Schizophrenia is thought to develop from very complex geneenvironment interactions with vulnerability factors. The interactions of these risk factors are intricate, as numerous and diverse medical insults from conception to adulthood can be involved. The combination of genetic and environmental factors leads to deficits in the neural circuits that affect sensory input and cognitive functions. Historically, this theory has been broadly accepted but impossible to prove given ethical limitations. The first definitive proof that schizophrenia arises from multiple biological changes in the brain was recently established in human tissue grown from patient stem cells, where the complexity of disease was found to be “even more complex than currently accepted” due to cell-by-cell encoding of schizophrenia-related neuropathology.
A genetic predisposition on its own, without superimposed environmental risk factors, generally does not give rise to schizophrenia. Environmental risk factors are many, and include pregnancy complications, prenatal stress and nutrition, and adverse childhood experiences. An environmental risk factor may act alone or in combination with others.
General Objectives For The Treatment Of Schizophrenia Are To
- Reducing the severity of psychotic symptoms
- Preserve psychosocial function
- Preventing recurrence of symptomatic episodes and associated functional impairment
- Reduce the use of recreational substances
The main components of treatment are antipsychotic medication, rehabilitation through social support services and psychotherapy.
Since schizophrenia is a long-term, recurrent disorder, teaching patients self-management techniques is a significant overall goal. Providing information about the disorder to parents of younger patients may reduce the relapse rate . .
Antipsychotic drugs are divided into conventional antipsychotics and 2nd generation antipsychotics based on their affinity and receptor activity to the specific neurotransmitter.
Second-generation antipsychotics offer some advantages both in terms of discretely greater efficacy and in reducing the likelihood of developing an involuntary movement disorder and related adverse effects.
However, the risk of developing a metabolic syndrome is greater with 2nd generation antipsychotics than with conventional ones.
Several antipsychotics in both classes can cause long QT syndrome and ultimately increase the risk of fatal arrhythmias these drugs include thioridazine, haloperidol, olanzapine, risperidone, and ziprasidone.
Don’t Miss: How Do Bipolar People Act When Drunk
Spectrum Disorders: How Broad Is The Range Of Psychiatric Illness Transmitted And Who Do We Consider Affected
In general, the risk for all psychotic spectrum disorders is increased in the relatives of schizophrenics. The risk of schizophrenia is also significantly higher in the relatives of individuals with spectrum disorders. Most studies suggest that relatives of schizophrenic patients are not at increased risk of anxiety or alcohol and drug dependence disorders. The results for bipolar affective disorder are less clear, but taken together suggest that schizophrenia and bipolar disorder might have both shared and independent genetic risk factors. It is therefore reasonably common to perform several analyses of data using a number of different definitions of illness.
Psychosocial Factors During Pregnancy And Delivery
Some studies suggest an association between antenatal stress and schizophrenia. The children of mothers whose husband died while they were pregnant have been found to have a significantly increased rate of schizophrenia compared with children who lost their father in infancy in the first year of life. In The Netherlands, rates of schizophrenia have been found to be very slightly higher in individuals exposed in utero to war and flood disaster than in reference subjects.
In the Northern Finland 1966 Birth Cohort the risk of later schizophrenia among unwanted children was elevated 2.4-fold compared with wanted or mistimed children, even after adjustment for confounding by sociodemographic, pregnancy and perinatal variables. Unwantedness might be a marker for features associated with risk in either the mother or the child. In the same cohort, the level of schizophrenia in the offspring of antenatally depressed mothers was elevated by a factor of 1.5-foldly, but the association was not statistically significant. Those mothers of schizophrenia patients with a psychotic first-degree relative had suffered from depressed mood during pregnancy twice as often as other mothers. The familial risk for psychosis, including genetic risk for psychosis, might explain the elevated prevalence of depressed mood during pregnancy among the mothers of the offspring who went on to develop schizophrenia.
You May Like: What Is The Phobia Of Death Called
Symptom Categories In Schizophrenia
Generally, symptoms are classified as
- Positive: a distortion of normal functions
- Negative: a decrease or loss of normal functions and affectivity
- Disorganised: disturbances in thinking and bizarre behaviour
- Cognitive: deficits in information processing and problem solving
Patients may experience symptoms in one or more categories.
Positive symptoms can be further classified as
Delusions are erroneous beliefs that are maintained despite clear contradictory evidence.
There are several types of delusions:
- Persecutory delusions: patients believe they are being harassed, followed, cheated or spied on.
- Reference delusions: Patients are convinced that passages from books, newspapers, song lyrics or other environmental stimuli are directed at them.
- Delusions of theft or thought graft: patients believe that others can read their minds, that their thoughts are being transmitted to others, or that thoughts and impulses are being imposed on them by external forces.
Delusions in schizophrenia tend to be bizarre, i.e., implausible and not derived from common life experiences .
Hallucinations are sensory perceptions that are not perceived by anyone else.
They may be auditory, visual, olfactory, gustatory or tactile, but auditory hallucinations are by far the most common.
Patients may hear voices commenting on their behaviour, conversing with each other or making critical and hurtful comments.
Delusions and hallucinations can be extremely irritating for patients.
Negative symptoms include
When Do Schizophrenia Symptoms Begin
Even though genetic and environmental factors are often present in early life, schizophrenia symptoms typically do not begin to show prominently until young adulthood.
Schizophrenia can occur at any age, but the average age of onset for men is late teens to early 20s. In women, symptoms generally appear in their late 20s to early 30s.
Schizophrenia appears to share genetic overlap with other common mental health conditions. Close relatives of people with schizophrenia may be at a higher genetic risk of developing bipolar disorder, epilepsy, and autism.
In one major 2018 study , an international research team looked at the data of more than 33,000 people with schizophrenia, 20,000 people with bipolar disorder, and 54,000 people without either condition.
Researchers found 114 specific loci that contribute to the risk of both schizophrenia and bipolar disorder. They also found four genome regions that distinguish the biological differences between the two conditions.
According to the study, it may be possible to determine whether a person is likely to develop certain symptoms of schizophrenia based on their individual genetic risk score for either condition.
For example, the study found that people with bipolar disorder with psychosis are likely to have a higher genetic risk score for schizophrenia than those who have bipolar disorder without symptoms of psychosis.
Recommended Reading: What Are The 4 Main Types Of Schizophrenia
Possible Causes Of Schizophrenia
Like pneumonia, which can be caused by various bacteria, viruses, or chemicals, schizophrenia probably has multiple causes, all of which affect the brain in related ways. Research suggests that both genes and environmental factors are involved in developing schizophrenia. While 1 out of every 100 people has schizophrenia, having a biological relative with schizophrenia increases a persons risk of developing this disorder. A person who has a genetically identical twin with schizophrenia has a 50% chance of having schizophrenia. A person with a sibling or a parent with schizophrenia has a 10% chance of having schizophrenia. Research is aimed at finding both the genetic factors that may put a person at increased risk for schizophrenia, and the environmental factors that may be involved. There is active and exciting research to find the genes that increase risk for schizophrenia. Three areas on various chromosomes have been linked to schizophrenia in more than one study however, the actual gene that increases risk for schizophrenia has not yet been found.
These and other studies hold promise for our eventual understanding of how genes and environment may interact to cause schizophrenia. Regardless, evidence is overwhelming that schizophrenia is a biologically based illness and that the previous view that parents or families cause schizophrenia is totally without merit.